The journey through the world of additives with condition monitoring specialist company, WearCheck, continues. This time, we dive into the bubbly world of foam inhibitors.
| What are they? | Methyl silicone and organic polymers |
| What do they do? | Prevent the formation of foam |
| How do they do it? | Reduce the surface tension of the air/oil interface allowing air bubbles to burst more readily |
Steven Lumley, technical manager for WearCheck, introduces us to a very specialised group of additives known as interfacial additives.
We will begin with anti-foam additives (also known as foam inhibitors). To understand the modus operandi of this underrated additive, we will first take you on a brief detour to discuss how air co-exists with oil, the resultant problems caused, an experiment that involves beer, a crustacean known as the deadliest gunslinger in the sea and, finally, we will explain how foam inhibitor additives work their magic.
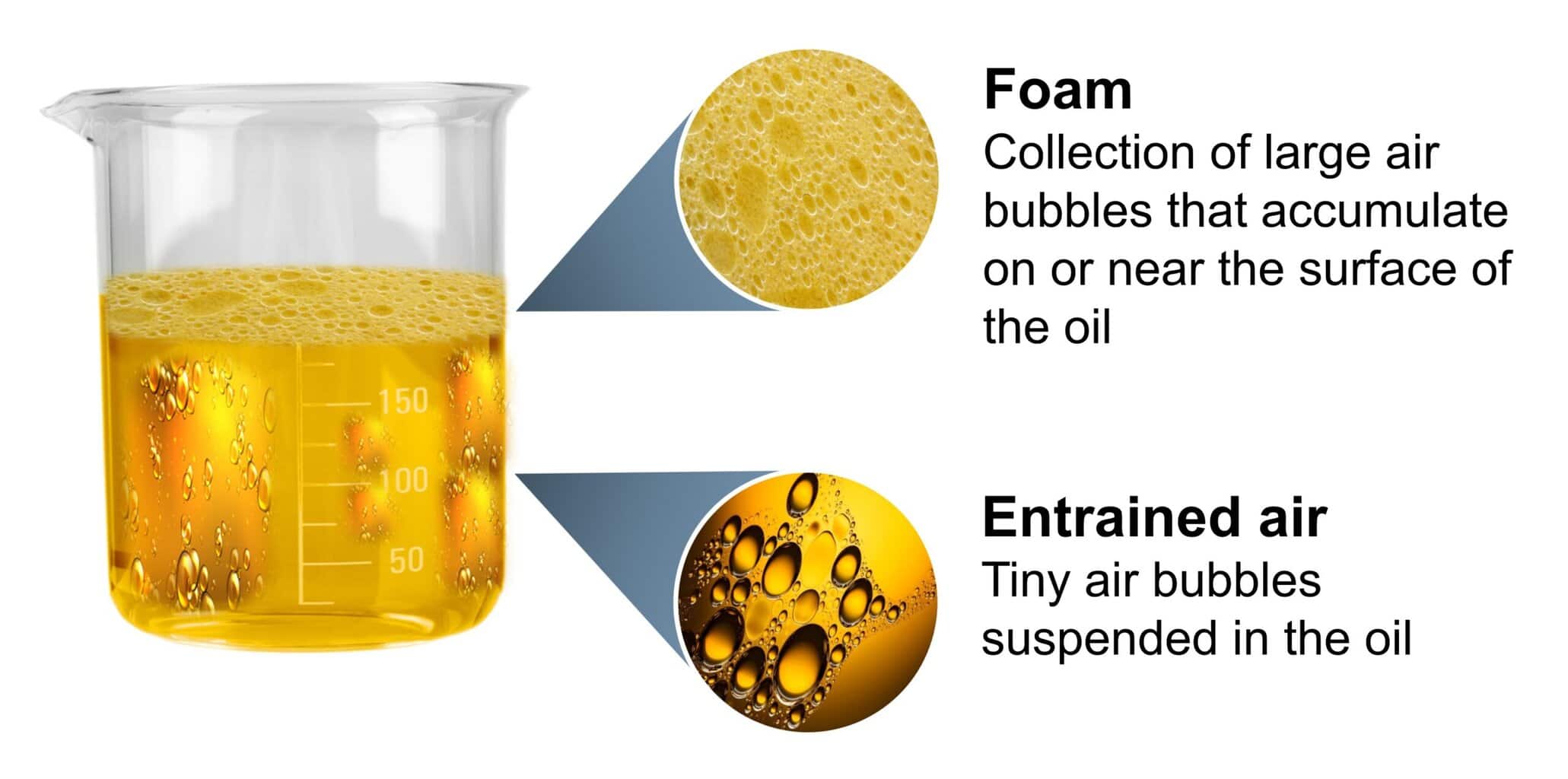
Air can exist in oil in three different states: dissolved, entrained and foam. Air dissolved in oil exists as individual molecules which are similar to CO₂ dissolved in soda water. Entrained air in oil is comprised of tiny air bubbles suspended in the oil. This type of air contamination is arguably the most damaging and can be identified by the oil having a cloudy appearance.
Finally, foam typically refers to the stable layer of relatively large bubbles that accumulate at the surface of a reservoir. In some systems, foam at the surface may not cause damage, but the presence of a foam layer normally indicates extensive air entrainment.
![]() When we think of contamination in lubricated systems, we often focus on particulate and water contamination and, in the case of engines, by-products of combustion – but the perils of air contamination are seldom given much thought.
When we think of contamination in lubricated systems, we often focus on particulate and water contamination and, in the case of engines, by-products of combustion – but the perils of air contamination are seldom given much thought.
While great in champagne, tiny air bubbles can have negative effects on lubricating oil and the mechanical system being lubricated. Air contamination can damage the lubricating oil by increasing the rate of oxidation and thermal degradation, depleting additives, reducing the oil’s heat transfer ability, reducing its film strength and can also cause gaseous cavitation.
Gaseous cavitation occurs when gas bubbles become compressed inside a pump or a cylinder. When pressure on the oil increases past the point where the bubbles can compress further, the bubbles implode. The resulting shock wave produces noise and vibrations which can cause excessive wear. Cavitation bubbles generally need a surface on which to nucleate. This surface could be the sides of the reservoir, contaminant particles in the oil, entrained air bubbles or roughened internal surfaces.
To test the theory of nucleation in bubble formation, try the following beer and peanut experiment as described by Noria:
Take a freshly poured glass of beer and drop a salted peanut into the glass. Watch as the weight of the peanut causes it to sink to the bottom of the glass. But keep watching! After 10 to 20 seconds, the peanut miraculously rises to the surface again. The reason is that the salt grains on the surface of the peanut act as nucleation sites for the growth of gas bubbles, in this case, carbon dioxide from the carbonization process. When the peanut reaches the surface, the gas bubbles detach from the surface of the peanut and once again, the peanut will sink to the bottom, where the process starts again. Depending on how much salt is on the surface of the peanut, this activity can continue for several minutes, until finally all the salt is washed away, and the peanut falls to its final resting place. Now isn’t that a science experiment worth trying . . . Cheers!
Air contamination can also lead to a phenomenon known as microdieseling which, despite its name, has nothing to do with diesel and the problems it creates are anything but micro in magnitude.
Microdieseling is a pressure-induced thermal degradation or, simply put, microdieseling occurs when gas bubbles become hot enough to ignite. An air bubble will transition from a low or negative pressure area to a high-pressure zone and, through adiabatic compression, get heated to very high temperatures. These temperatures are high enough to carbonise oil at the bubble interface, resulting in carbon by-products (sludge and varnish) as well as increased oil degradation (oxidation), higher operating temperatures, pressure spikes and cavitational erosion of hydraulic pump and other components.
Still not convinced that a teensy-weensy air bubble can be a problem? Well, say hello to my little friend, Synalpheus Fritzmuelleri – AKA the pistol shrimp – who has earned the title of the deadliest gunslinger in the sea.
Being called a shrimp doesn’t exactly make you known for having formidable strength or an intimidating presence, but the pistol shrimp crushes this stereotype in a dramatic way and, more relevantly, perfectly demonstrates the destructive power of gaseous cavitation and microdieseling.
The piston shrimp Is approximately 3-5cm in length and shoots out a cavitation bubble from its enlarged snapping claw to kill its prey by stunning. The bubble travels at a sprightly 100Km/h and when it collapses reaches sound levels of 218dB. Just for context if you were standing 30m away from a running jet engine it would produce sound levels of 150 dB. The collapsing cavitation bubble reaches temperatures of 4720 ˚C.
A lesser known interesting fact for those budding WWII historians out there – The sound created by the piston shrimp threw an unlikely wrench into the allies’ defence plans during World War II. The ‘snap-crackle-pop’ sound produced by this formidable marine creature began interfering with sonar used by the allies to detect enemy ships. This prompted the U.S. navy to enlist the help of marine biologists and acoustical physicists from the University of California’s division of War Research. Fortunately, they were able to record the shrimp’s sounds to train sonar operators to recognise them as sea denizens rather than enemies and the rest, as they say, is history.
Beer experiments and dangerous crustaceans aside, let’s finish off this segment with the star of the show – the additive that bursts your bubble.
Foam inhibitor additives work their magic at the air-oil interface and that is why we refer to them as an interfacial additive. These additives are surfactants in that they reduce the surface tension of a liquid in which they are dissolved. The chemicals in this additive group possess low interfacial tension, which weakens the oil bubble wall and allows the foam bubbles to burst more readily.
Oil-insoluble silicone-based foam inhibitor additives, which are the most widely used in lubricant formulation, are not dissolved, but rather dispersed finely in the lubricating oil and very low concentrations (typically blended into the base oil at 5-20ppm) are usually required. If too much foam inhibitor additive is added, it can have a reverse effect and promote further foaming and air entrainment – this really is a case of too much of a good thing is bad!
Visit www.wearcheck.co.za or email marketing@wearcheck.co.za for more information.